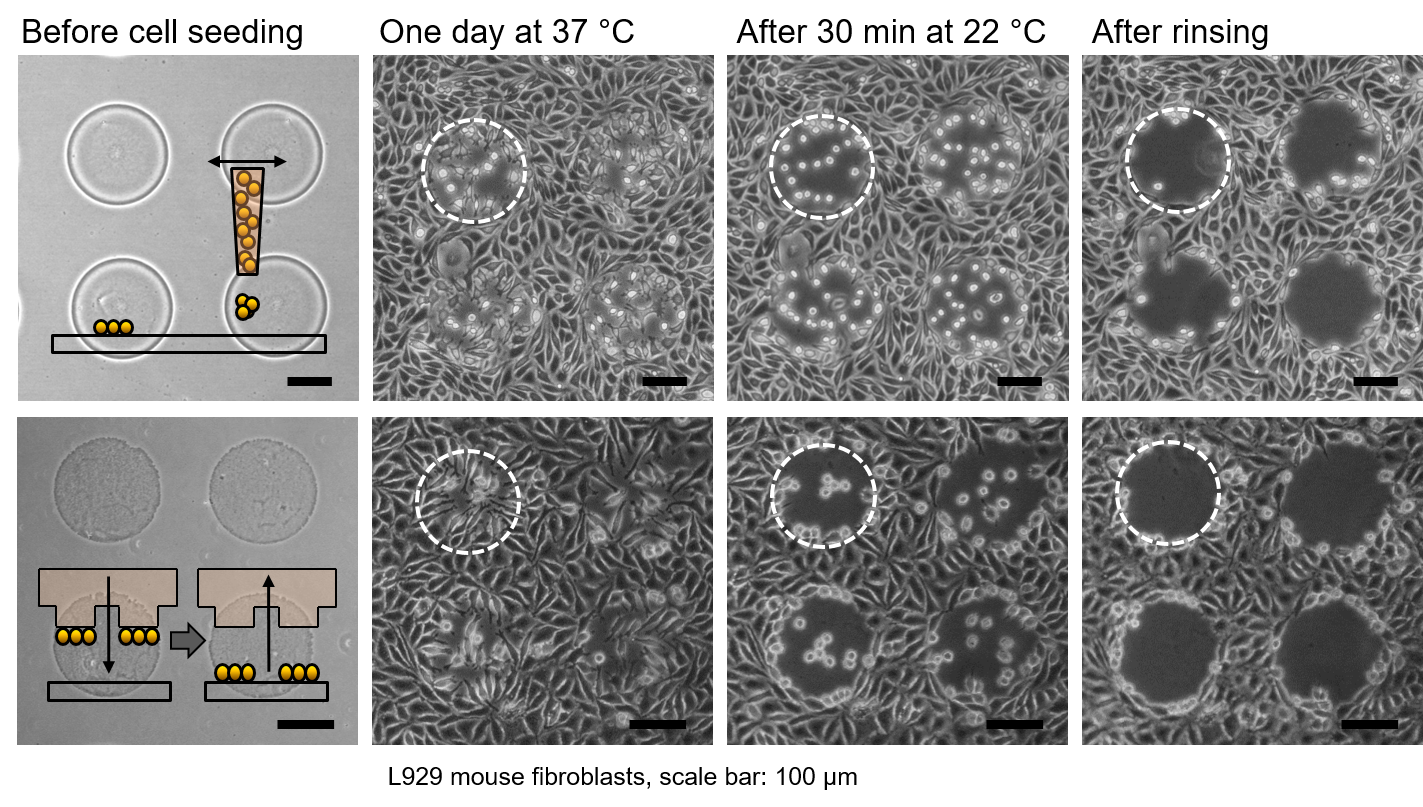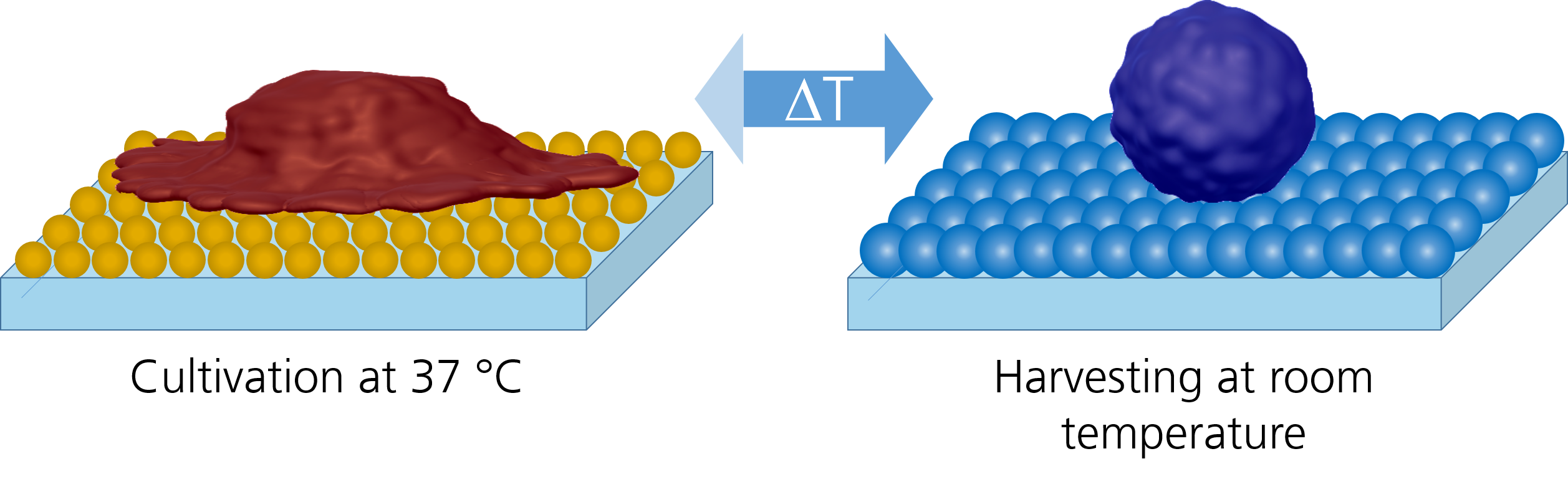
We develop coatings of thermoresponsive polymers for cell culture applications with the aim of effectively and gently controlling cell adhesion. At typical cultivation temperatures, cells adhere and proliferate as on a standard cell culture substrate. If the temperature is reduced by a few degrees, the cells can be detached from these coatings by simple rinsing step. The absence of invasive proteases ensures that cell viability and membrane proteins are not compromised during this critical process step. The polymers can be applied homogeneously or in defined patterns to common cell culture substrates at low cost using simple methods such as spin coating, spray coating, spotting or printing. In addition to use as a cell culture substrate, the polymer coating is suitable for cell tests that allow cell migration to be investigated (e.g. wound healing test) or for establishing co-cultures with defined geometric relationships.
 Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB
Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB