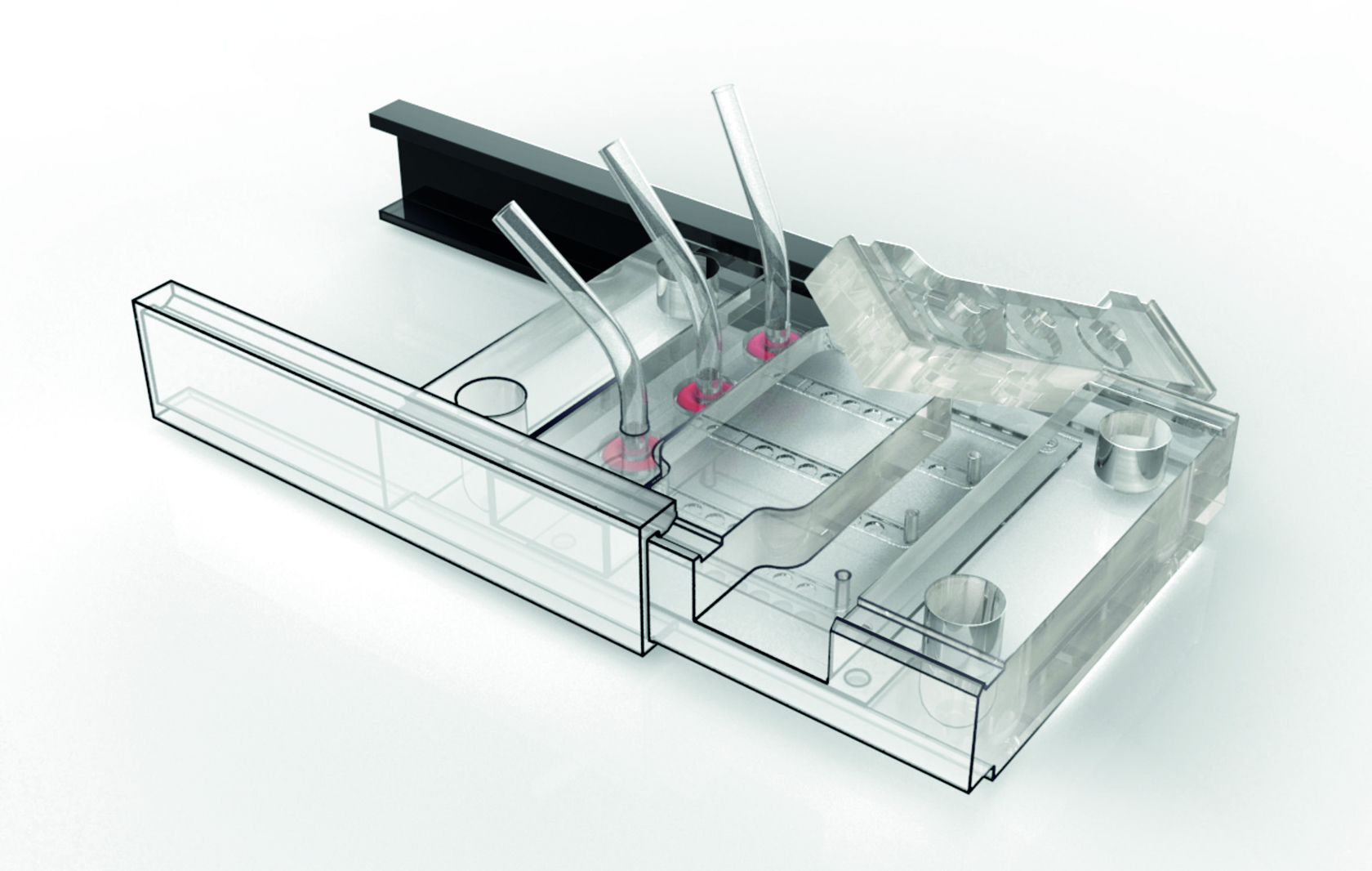
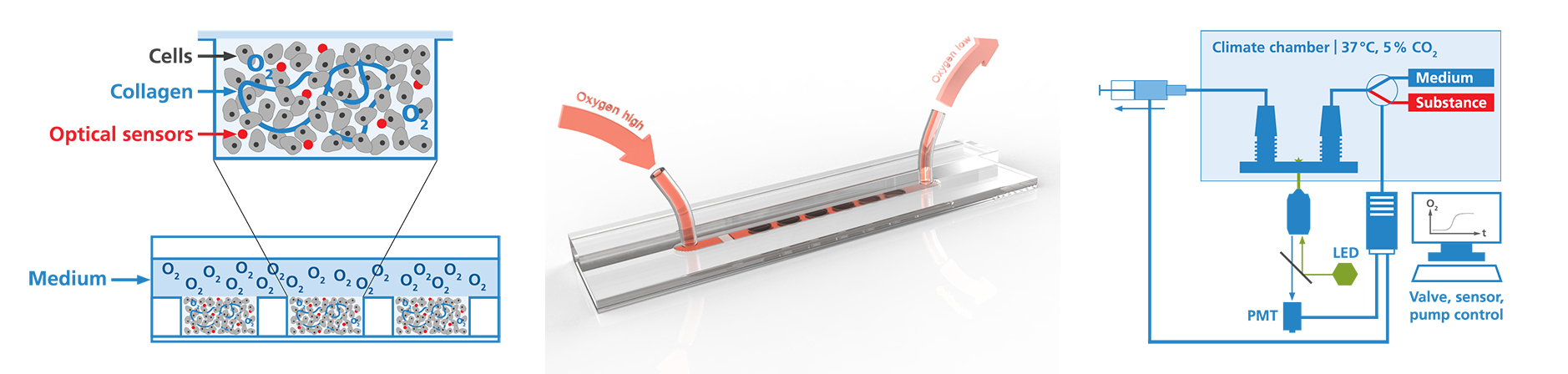
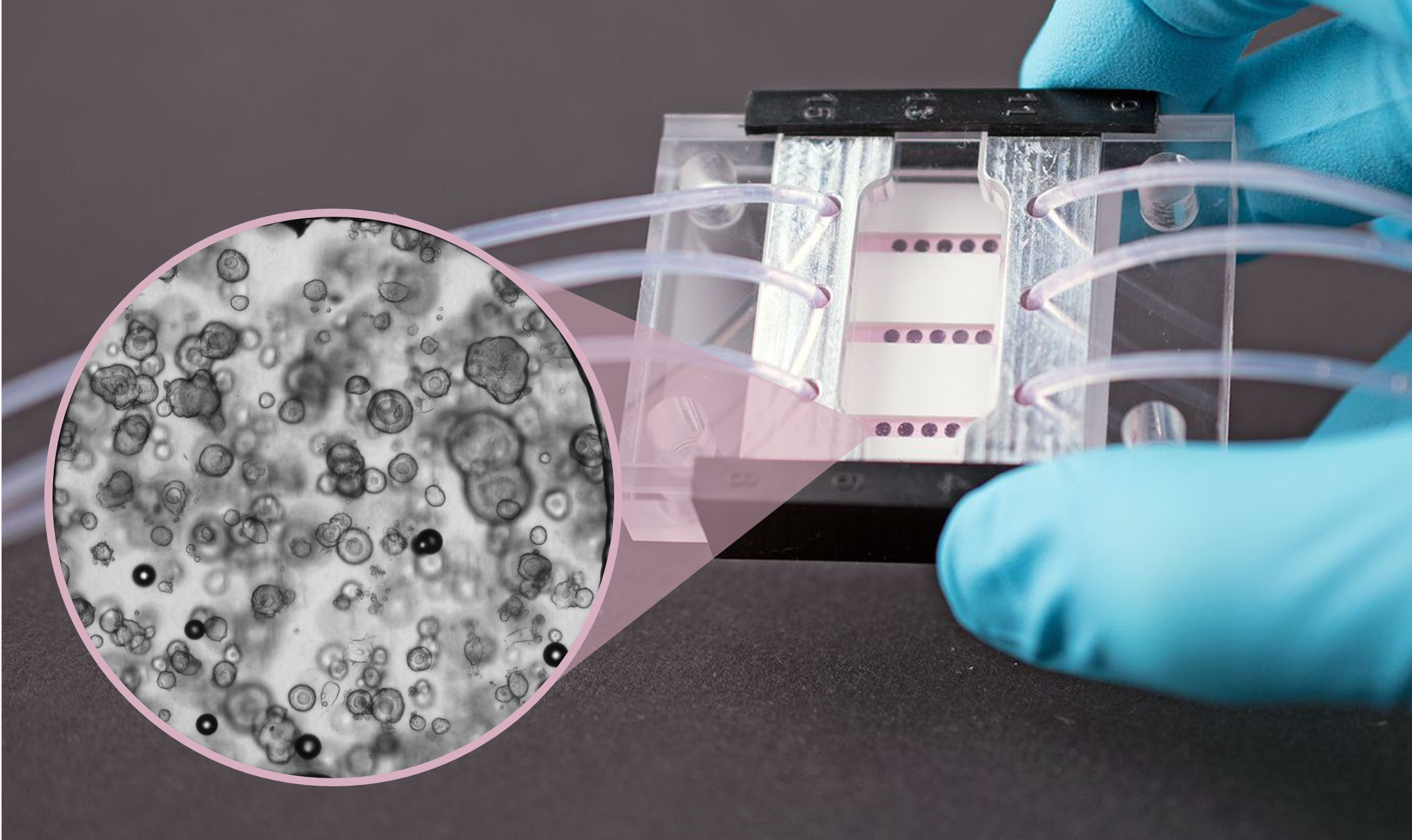
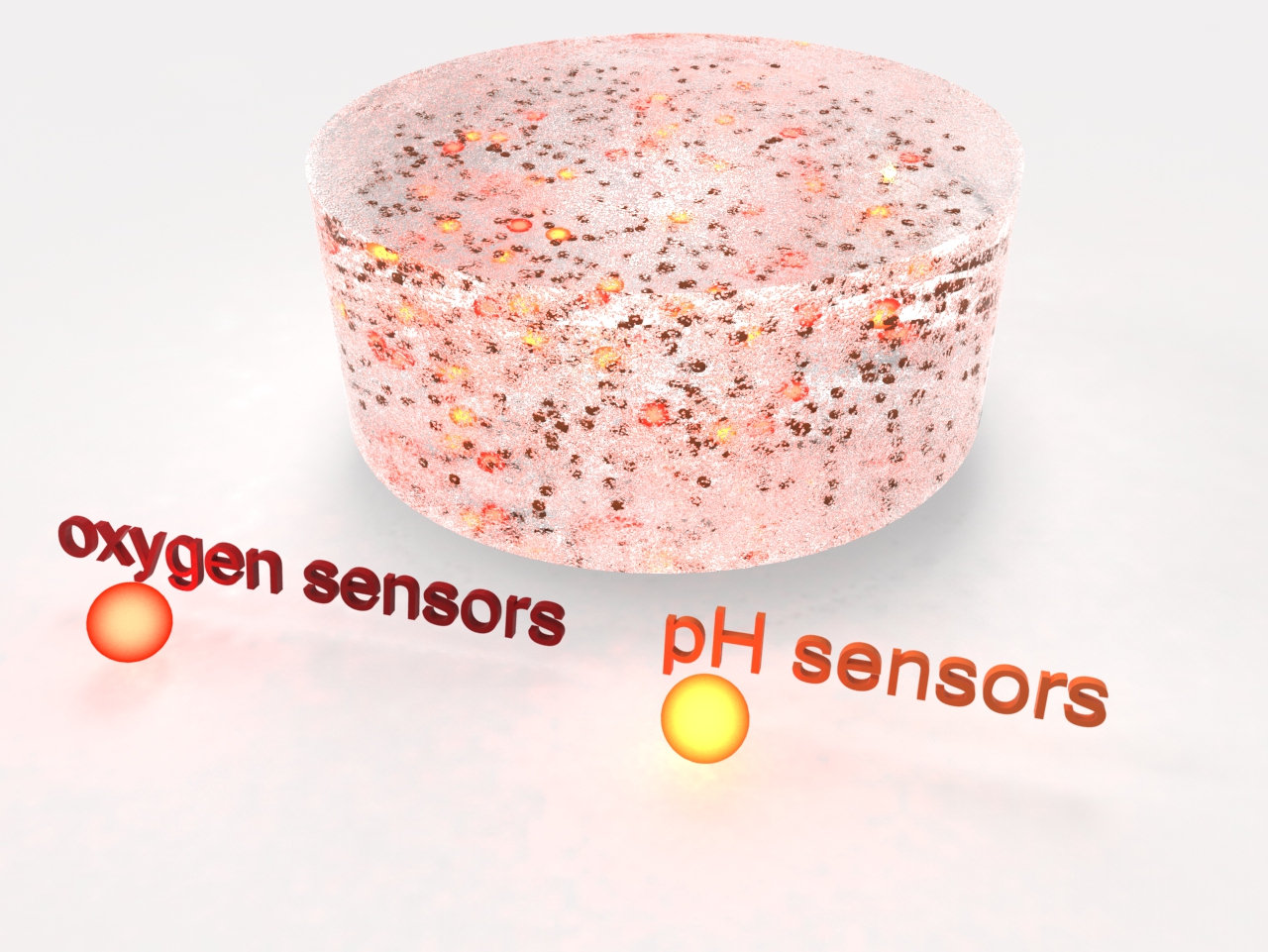
Animation of cell seeding and assembly of the organ-on-chip system.
In vitro cell models are used as disease models in drug screenings and toxicity tests and in basic research to develop and organize various tissue types. Based on our expertise in cell biology, material sciences and coatings, as well as microsensors and microfluidics (design, manufacturing and integration), we develop microreactors for short- and long-term studies on cell tissue. These reactors ensure a cell culture under controlled physiological conditions. By integrating microsensors into these systems, we can for the first time extend continuous real-time measurements of cell vitality or metabolites to up to one month. Compared to conventional endpoint measurements, this approach provides a much more in-depth and direct access to the understanding of drug mechanisms. In addition to the design and development of microbioreactors, we realize in vitro studies to evaluate the liver toxicity of chemical substances.
 Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB
Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB